WP1: Project management
Eelco Soeteman
Netherlands Heart Institute
- Folkert Asselbergs – DCVA /Amsterdam UMC
(Consortium Leader) - Dennis van Veghel – NHR
(Consortium Leader) - Saskia Houterman – NHR
- Alicia Uijl – Amsterdam UMC

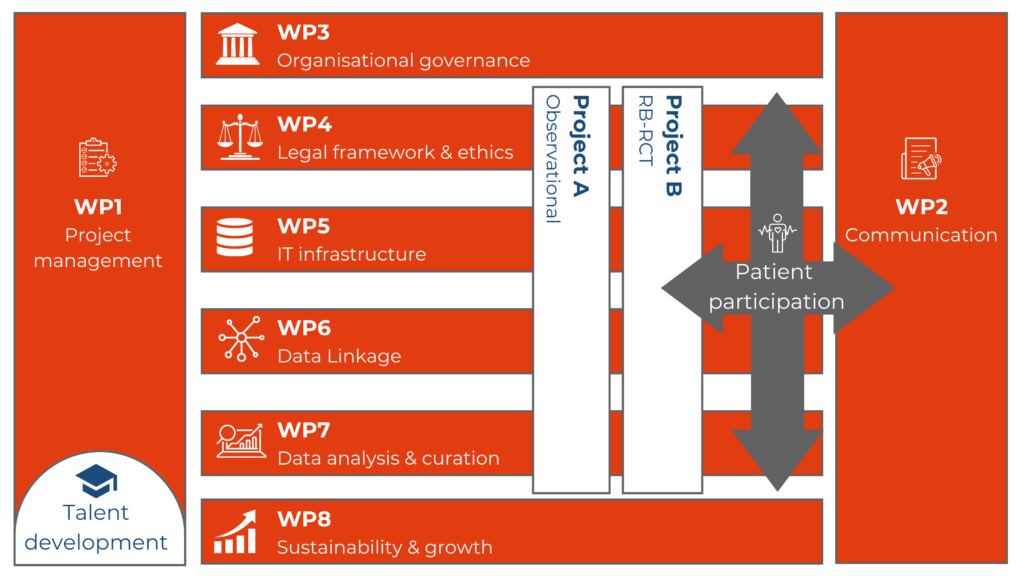

WP2: Communication
Noud de Greef
NHR
- Marjolein Snaterse – NVHVV
- Pjotr van Lenteren – Hartstichting
Objective
Interaction and collaboration with different internal and external partners is an important priority of the HEART4DATA consortium and will be the main responsibility of WP2. In WP2 the internal and external communication of the consortium and the dissemination of the consortium results is described and executed. The DCVA Health Data Hub will be established.
Stakeholders will be engaged via the User Committee. Furthermore communication with the stakeholders will take place via WP2.
One of the main strengths of the HEART4DATA consortium are the diverse partners and stakeholders involved, these partners will have a large and diverse outreach via their respective channels which comprises not only an academic and scientific community, but also industry, patients and the general public. Therefore,
- a list will be created with all communication channels of DCVA partners with corresponding public.
- the HEART4DATA consortium will have a standard introduction/explanator slide deck and video.
The internal communication within the consortium is focussed on the different consortium partners that are involved using progress reports, aligning consortium partners using the different committees and organisations and stimulating using the infrastructure.
The external communication is focussed on dissemination of this initiative and the generated results of the consortium within two dimensions. One to e.g. researchers, patients, healthcare workers and the other to relevant stakeholders not embedded within the consortium.
DCVA Health Data Hub
An important goal of the dissemination strategy is to stimulate engagement of all different stakeholders in the cardiovascular field within and outside the consortium.
Achievements & Milestones
- Overall communications plan
- Heart4Data LinkedIn
- Explanation animation
- Manual for communication from project- and researchpartners

WP3: Organisational governance
Jolien Roos
DCVA
Rudolf de Boer
NVVC
- Dennis van Veghel – NHR
- Saskia Houterman – NHR
- Martin Hemels – WCN
- Ron Pisters – NVVC Connect
- Eelco Soeteman – NLHI
- Edgar Daeter – NHR Registratiecommissie cardiochirurgie en NVT
- Wilson Li – NVT
- Rebecca Abma Schouten – Hartstichting
Objective
The objective of this work package 3 is to develop and implement the governance structure regarding initiating and continuing cardiovascular registry-based research in the Netherlands.
The first goal is to develop a sustainable governance structure supported by all DCVA partners together with funding strategies (the DCVA fund) for maintenance of the infrastructure and future research projects. The primary focus is on heart failure. After that other cardiovascular domains will be included. The second long-term goal is to have a sustainable funding system for maintenance of cardiovascular registries within the NHR.

WP4: Legal framework & ethics
Susanne Rebers
Health RI
- Astrid Schut – WCN
- Eelco Soeteman – NLHI
- Saskia Houterman – NHR
- Anton Ekker – NHR
- Jan Tijsen – Cardialysis
Objective
The objective of this work package 4 is to create a separate legal and ethical framework for all types of registry-based research, including observational studies with real-world data and RB-RCTs.
- The legal framework will be compliant with the legislation in the Netherlands according to the Healthcare Quality, Complaints and Disputes Act (WKKGZ), the Care Quality Registration Act (Wkz), the Medical Treatment Agreement Act (WGBO), the Medical Research Involving Human Subjects Act (WMO) and the General Data Protection Regulation (GDPR; in Dutch AVG).
- A flow chart for the different types of registry-based research will be created for the legal framework. Different standard contracts are drawn up for registry-based research needed within the different work packages like clinical trial/study agreement and data/material transfer agreement.
- The legal and ethical framework will be implemented within the NHR in such a way that registry-based research within the NHR It infrastructure is possible.

WP5: IT infrastructure
Dick Schuurman
Nederlandse Hart Registratie
- Erik van Iperen – HealthRI/Durrer Center
- Saskia Houterman – NHR
- Emile Brinkman – AmsterdamUMC/KIK
- Anita Ravelli – AmsterdamUMC/KIK
- Andre Dekker – HealthRI
- Aiara Lobo Gomes – Maastricht University
Objective
The objective of this work package 5 is to create an operable IT infrastructure for registry-based research. Activities:
- The IT infrastructure will be embedded within the NHR using the NHR registries
- Education modules should be developed about using the IT infrastructure
- An expert group with cardiologists, researchers, nurses and data managers should be composed for testing the different components of the infrastructure.
- Different data sources for gathering the (additional) registry-based data can be used, like bulk uploads from the EHR, manual eCRFs within the NHR or eCRFs from external parties.
- For data linkage with data from external sources, such as PHARMO or DHD, bulk uploads will be facilitated or data will be forwarded.
- Gathering data about PROMs and PREMs of patients is one of the other important topics within this work package.
- One of the activities within this work package is reducing the data registration burden in the hospitals through enabling bulk uploads from the different EHR systems within the hospitals.
- A public dashboard for publishing generic data in collaboration with Hart & Vaatcijfers and a portal for researchers to apply for data will be developed together with the DCVA infrastructure pillar.
Achievements & Milestones
- Mergetool for dataflow from TITRATE-HF to NHR HF registry
- 22 may 2023: Reached 2000 included patients thanks to synergy with TITRATE-HF

WP6: Data Linkage
Andre Dekker
Health RI
- Evelien van de Schaaf – HealthRI/Durrer Center
- Saskia Houterman – NHR
- Alicia Uijl – Amsterdam UMC
- Marco Alings – Julius Clinical
- Aiara Lobo Gomes – Maastricht University
- Pim Volkert – HealthRI
- Niels Bolding – HealthRI
Objective
The main objectives of WP6 are 1) to link reusable data from different data sources with the NHR registry and 2) to make the national HF registry interoperable with other HF registries, electronic patient records and international initiatives through a common data model.
- The primary activity of WP6 is investigating the potential linkage of the national HF registry with different data sources for registry-based research. The first goal of WP6 is to set up sustainable data linkage agreements with at least one data source to enrich the NHR in the first year of the HEART4DATA consortium. In the first phase we will set up data linkage agreements with PHARMO and CBS. In the first year we will conduct a pilot study embedded in project A to assess the possibility of data linkage with PHARMO. During the pilot study a decision model will be made to agree upon data pairing, data cleaning and linkage quality.
- The second aim is to embed titration studies within the NHR using PHARMO linkage. In the following years sustainable data linkage agreements, following the same principle as linking with CBS and PHARMO, with other data sources will be established. Therefore, the legal, financial and logistic part for linkage with these specific data sources should be investigated.
- In addition, this work package will work on mapping data from different sources with common data models for comparative research on both national and international level. A common data model is a model to harmonise data between different data sources in a common, standard format.
- Lastly, in phase 2 we will create a working plan for federated data. Together with Health-RI and other national initiatives such as SKR, we will create a strategy for federated data analysis.
Achievements & Milestones
- 22 june 2023: Data linkage CBS realised. Press release: NHR & CBS combine forces

WP7: Data analysis & curation
Eric Boersma
CBG / Erasmus MC
- Jacoline Kooistra – WCN
- Lineke Derks – NHR
- Michelle van der Stoel – NHR
- Stephane Heijmans – MUMC+
- Lennie Derde – UMCU
- Roland van Kimmenade – RadboudMC
- Charlotte Onland – UMCU
- Alicia Uijl – Amsterdam UMC
- Valery Lemmens – IKNL/ErasmusMC
- Laura Rodwell – RadboudMC
- Mira Zuidgeest – UMCU / Julius Clinical
- Peter van de Ven – UMCU
- Joost van Rosmalen – UMC Utrecht
- Astrid Schut – WCN
Objective
The main objectives of WP6 are 1) to link reusable data from different data sources with the NHR registry and 2) to make the national HF registry interoperable with other HF registries, electronic patient records and international initiatives through a common data model.
- The primary activity of WP6 is investigating the potential linkage of the national HF registry with different data sources for registry-based research. The first goal of WP6 is to set up sustainable data linkage agreements with at least one data source to enrich the NHR in the first year of the HEART4DATA consortium. In the first phase we will set up data linkage agreements with PHARMO and CBS. In the first year we will conduct a pilot study embedded in project A to assess the possibility of data linkage with PHARMO. During the pilot study a decision model will be made to agree upon data pairing, data cleaning and linkage quality.
- The second aim is to embed titration studies within the NHR using PHARMO linkage. In the following years sustainable data linkage agreements, following the same principle as linking with CBS and PHARMO, with other data sources will be established. Therefore, the legal, financial and logistic part for linkage with these specific data sources should be investigated.
- In addition, this work package will work on mapping data from different sources with common data models for comparative research on both national and international level. A common data model is a model to harmonise data between different data sources in a common, standard format.
- Lastly, in phase 2 we will create a working plan for federated data. Together with Health-RI and other national initiatives such as SKR, we will create a strategy for federated data analysis.

WP8: Sustainability & Growth
Jasper Brugts
RC AF / Erasmus MC
Ron Pisters
RC AF / Rijnstate / NVVC Connect
- Andre Dekker – HealthRI/Universiteit Maastricht
- Lineke Derks – NHR
- Rik van de Kamp – NHR
- Marjolein Snaterse – NVHVV
- Jose Henriques – NVVC
- Olivier Manintveld -NVVC HF working group
- Nelson Dapaah – Harteraad
- Jan van Ramshorst – NVVC Connect
- Peter Mol – CBG
- Marjon Pasmooij – CBG
- Sander de Groot – VIG
Objective
The primary objective of this work package is to create a strong basis for the long-term continuation and growth of the HF and AF registries within the NHR with a care pathway embedded in the electronic health record (EHR) systems and supported by all involved stakeholders. The registry should be large and of high quality and be representative of Dutch HF and AF care. By 2026 more than 40 hospitals will be contributing data and 15.000 HF patients will be included in the registry.
There are key necessities and activities to create a strong basis for sustainability and growth of the HF and AF registry for a large and high quality registration (>15.000 HF patients in 2026), which continues to grow even after 2026 and be representative for Dutch HF and AF care (>40 hospitals participating in 2026).
For the primary objective to be successful, the HF and AF registry should be:
- endorsed from all involved key stakeholders
- build in a stepwise fashion allowing participation from the start
- enriched by efficient data linkage to other relevant databases
- using synergies from major opportunities
Achievements & Milestones
- Mergetool for dataflow from TITRATE-HF to NHR HF registry
- 22 may 2023: Reached 2000 included patients thanks to synergy with TITRATE-HF

Project A: Guideline directed medical therapy (GDMT) adherence
Jasper Brugts
Erasmus MC
- Alicia Uijl – Amsterdam UMC
- Marish Oerlemans – UMCU
- Stefan Koudstaal – Groene Hart Ziekenhuis
- Kevin Damman – UMCG
- René Tio – Catharina Ziekenhuis
- Frederique Hafkamp -Nederlands Hartnetwerk
- Sandra Sanders-van Wijk – Zuyderland
- Gerard Linssen – ZGT
- Mieke van den Heuvel – MST
- Loek van Heerebeek – OLVG
- Carlos da Fonseca – MCL
- Vanessa van Empel – MUMC+
- Jan Willem Borleffs – HagaZiekenhuis
- Jan van Ramshorst – NWZ
- Hans-Peter Brunner-LaRocca – MUMC+
- Louis Handoko – UMC Utrecht
- Jeroen Schaap – WCN/Amphia
Objective
To use and prove value of the infrastructure by an observational, longitudinal research on the entire spectrum of patients with heart failure (including patients with HFpEF) in the Netherlands with focus on guideline recommended diagnostic trajectories and treatment.
The analysis in this project will focus on three main topics: patient subgroups, prognosis and quality of care. Patient subgroups will focus on HFpEF, including current treatment and phenotype clustering to reshape and refine disease entities. Also included here are sex related differences in HF and elderly, specifically differences related to suboptimal treatment and comorbidities.
Prognosis will be investigated in relation to NYHA class, ejection fraction and will consist of both mortality and morbidity outcomes: all-cause mortality, (non) cardiovascular mortality and hospitalisation and HF hospitalisation. Uptake of new recommended drugs will also be studied, including health technology assessment of current and new treatments. Quality of care is related to GDMT adherence, the challenges in achieving target dose and the relation between target dose and outcome measures.
Synergy with other work packages and initiatives
Before starting observational data analysis, certain prerequisites must be fulfilled for the HF registration. One of the aims of this project is to motivate the HF teams, balancing the workload/use of resources and minimal requirement of technical expertise with the ENGAGE-HF study.
Project A will make sure there is synergy with other registration studies, including TITRATE-HF and SELEQT-HF. In addition, it will streamline with other work packages to improve data extraction and upload processes and establishing links to other data sources, such as cause of death. Project A is looking for synergy with national and regional collaborative opportunities, including Health-RI, to support electronic health record-based registration.
ENGAGE-HF
Part of project A is the development of an engagement tool to promote the ease of registration and stimulate intrinsic motivation of caregivers with a competitive element to promote data entry (inclusion numbers) and data completeness (data quality).
This tool is aimed at heart failure nurses and deploys “gamification” and “engagement” in learning to make optimal use of quality registrations and treatment guideline implementation.

Project B: Seleqt-HF
Jeroen Schaap
WCN / Amphia
Peter van der Meer
UMCG
- Roland van Kimmenade – Radboud UMC
- Louis Handoko – UMC Utrecht
- Stefan Koudstaal – Groene Hart Ziekenhuis
- Kevin Damman – UMCG
- Frederique Hafkamp – Nederlands Hartnetwerk
- Hans-Peter Brunner-LaRocca – MUMC+
- Pim van der Harst – UMCU
- Sandra Sanders-van Wijk – Zuyderland
- Gerard Linssen – ZGT
- Mieke van den Heuvel -MST
- Jasper Brugts – Erasmus MC
Objective
To use and prove value of the infrastructure by a prospective randomized clinical research on pharmaco-therapeutic treatment in patients with chronic heart failure.
Despite improvements in treatment, heart failure (HF) still carries a substantial risk of morbidity and mortality. Currently, 240,000 patients are living with HF in the Netherlands. Further refinements in care are needed and acceleration of research findings into clinical practice is warranted to improve outcome. The digitisation of healthcare provides the opportunity to enable a learning healthcare system through re-using data from routine clinical practice, which could shorten the innovation loop from research to practice.
The aim of the Heart4Data consortium is to create a national and sustainable infrastructure for cardiovascular electronic health record (EHR)-based, registry-based research in the Netherlands. SELEQT-HF will be the first registry based randomized clinical trial (RB-RCT) to test the infrastructure as proof-of-concept study.
The proposed infrastructure will be tested in a low-risk RB-RCT with respect to patient-safety. Therefore, we choose to study the clinical benefit of Selenium/CoQ10, a dietary supplement, in patients with HF. Selenium/CoQ10 supplements are safe and have no known side effects and can therefore be given to all patients presenting with HF. Here, the effect of Selenium/CoQ10 treatment on repeated HF hospitalizations and cardiovascular death in patients with HF will be evaluated.

Funding
The DCVA consortium Heart4Data has been made possible thanks to a grant from ZonMW and the Dutch Heart Foundation.



